| Subculture | Columbia blood plate (English name: CA-B): casein pancreatin digest 10.0g, cardiopancreatin digest 3.0g, corn starch 1.0g, meat gastric enzyme digest 5.0g, yeast extract 5.0g, sodium chloride 5.0, agar 20.0g, distilled water 1.0L,pH 7.3±0.2. After sterilization at 121 ℃ for 15min, cool to 55 ℃, add 5% sterile defibrated sheep blood, shake well, pour the plate and cool it for later use. |
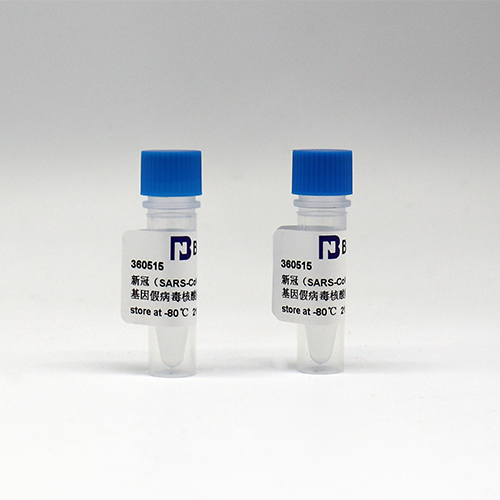
| Subculturing | Take it out of the refrigerator, and after it is completely melted, the vortex oscillates and mixes well, and it can be used. Note: The essential control is inactivated particles, which need to be used after nucleic acid extraction. The extraction or purification reagents should be provided by oneself, and repeated freezing and thawing should be avoided before extraction. The minimum sampling amount is 200 μl. |
| Growth conditions | 37 °;3-4 days; 5% CO2; |
| Storage conditions | -80 ℃ |
| Safety level | 2 |
| Isolation | Human gastric antrum, Australia |
| Application | The essential control product is the suspension of Helicobacter pylori inactivated bacteria. By extracting nucleic acid, using fluorescence quantitative pcr and digital PCR value, it can be used for method establishment, work standard assignment, method confirmation and evaluation, product quality control, etc. |
| Sharing mode | Public welfare sharing |

 info@weiyel.com
info@weiyel.com
 - English
- English
 - Русский язык
- Русский язык